Not only in corona times: When filling sterile injectables such as vaccines, the integrity of the primary packaging and the safety of its valuable contents are crucial. Straight-line inspection systems from Heuft inspect them inline and now cover the entire range with a wide variety of detection technologies.
Like everything that is injected, vaccines must be just as uncontaminated, highly pure and safe be the same as the primary packaging from which they are administered parenterally, i.e. „past the stomach“. The risk of primary packaging affected by cracks and other cosmetic defects, which does not seal tightly and may therefore be microbiologically contaminated, is unacceptable. Glass splinters, metal particles, hair, fibres and other foreign particles, which are often carriers of fungi or germs, should also not be overlooked.
For filled ampoules or blow-fill-seal containers that are sealed by melting, Annex 1 of the GMP guidelines has therefore long required a 100 per cent inline control in the sterile manufacturing and packaging process. It is also becoming apparent that container closure integrity testing (CCIT), as recommended in USP Chapter 1207 of these Good Manufacturing Practices, will become mandatory for vials in the near future, not only on a random basis, but also for each individual full container. In addition to the visual final inspection, where this is already the case, no filled injection vial may be left out.
Closure inspection and head-space analysis
Of fundamental importance for the functionality of the small vials and a safe injection is above all the Closure integrity. Subsequent contamination must no longer compromise the microbial purity of the active pharmaceutical content. With a detection unit specially designed for use in hygienically demanding laminar flow areas for reliable stopper seat inspection and powerful FinalView II CAP technology, which inspects crimped and flip-off caps including all their safety elements from all sides, the leading provider of straight-line inline inspection solutions has exactly the right optical modules to reliably remove incorrectly sealed, potentially leaking vials from circulation. Dented, incorrectly crimped, incorrectly seated, defective and foreign closures are reliably detected.
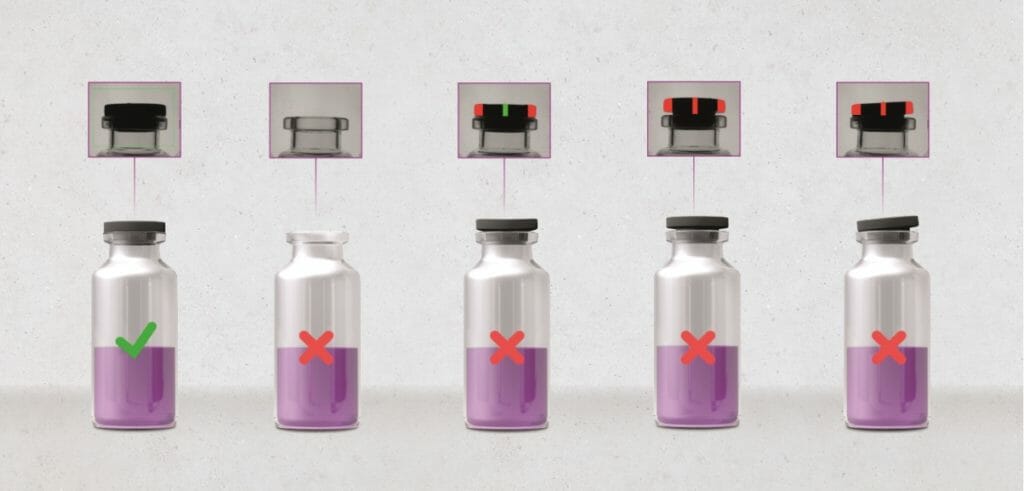
The stations for this camera-based closure inspection can be integrated into the compact spotter II PHS full-vial inspector. This also applies to the recently added module for Head space analysis, which complements and completes the CCIT. Using laser technology, it identifies leaking vials that have too much oxygen inside them. This can cause oxidation and impair the drug's mode of action.
Pulsed X-ray technology for full detection reliability
The spotter II PHS also optically detects deviations in fill quantity and product colour, impurities, „product splashing“ and glass defects in the vial as well as low-density foreign particles that have sunk to the bottom of the container. And with pulsed X-ray technology, which Heuft offers exclusively, even in non-transparent freeze-dried preparations Hidden foreign objects of high density, such as glass splinters in particular, are visualised gently and precisely.
The image processing developed in-house distinguishes between real and supposed faults and thus ensures Full detection and rejection reliability. Thanks to their particularly compact dimensions, the unique X-ray flash modules of the new generation can be integrated into the tightest of spaces - in some cases even directly into the respective packaging or converting machine.
Pre-filled syringe inspection with new needle hook detection
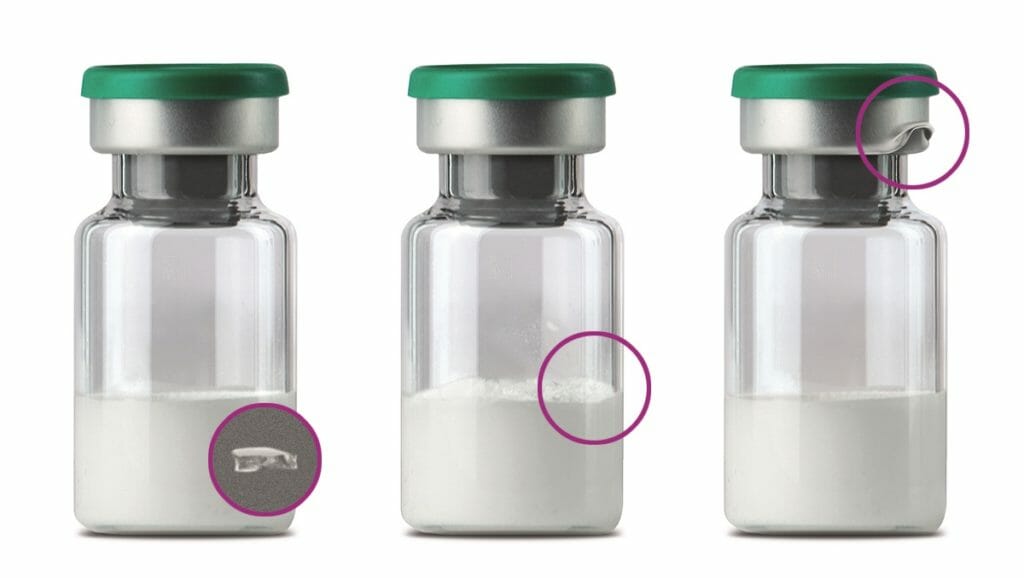
A good example of this is the Syringer module, which checks the functionality and safety of prefillable prefilled syringes. They are increasingly being used to administer vaccines and other injection preparations, among other things to avoid the risk of incorrect dosages. And pulsed X-ray inspection eliminates further sources of danger by identifying protective caps punctured by the injection needle, such as soft or rigid needle shields, as well as bent and deformed needles or incorrectly assembled, unsafe to use, defective or leaking Luer lock screw adapters and tamper-evident closures on such disposable syringes.
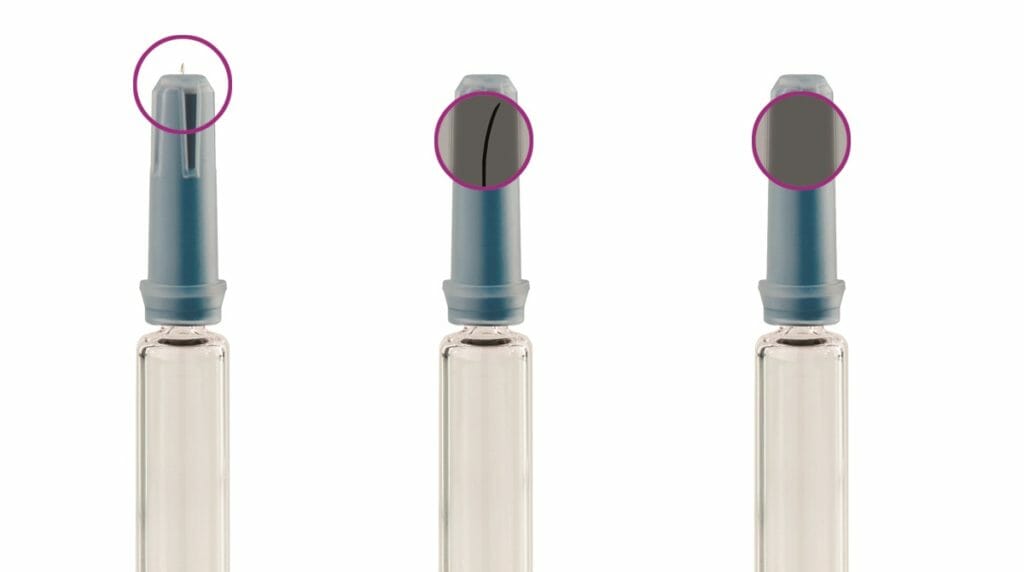
Micrometre-sized deformations at the tip of the cannula pose an additional risk. They are barely recognisable to the naked eye and radiometrically. This is why the Syringer now also has innovative additional optics that optimally complement the pulsed X-ray inspection: even before the protective covers are put on, it uses an intelligent colour sensor camera developed in-house to examine the tiny needle tips from above. The special light scattering of the adaptive LED lighting used for this purpose ensures that even smallest formal errors become visible.
Inspecting the primary packaging of injectables: timely empty vial inspection
The current Heuft solution for 100 per cent inline inspection, which is currently being installed in a new German production line for Covid-19 vaccines, is also camera-based in order to Faulty injection vials, contaminated with splinters due to possible glass breakage in the hot sterilisation tunnel, nor before the actual filling process to detect. This is all the more important because vaccine is a rare, valuable commodity where every single dose is important: if foreign objects and damage are only identified after filling, several of them will be irrevocably lost. This is because the affected vials and their contents must then be removed and disposed of.
Instead, in order to remove them from circulation before the valuable product enters, the directly above the filling machine inlet starwheel the smart sensor camera with special lighting and directly integrated image processing from Heuft. This top-down inspection system is able to detect glass splinters as well as other impurities, cracks and defects at the bottom of the vials. The position of the affected containers is precisely tracked so that they cannot be filled with the liquid vaccine under any circumstances. In the plant, which will soon 12,000 vaccine doses per hour are produced and packaged under aseptic conditions, a 100 per cent inspection of every single empty vial is guaranteed.
Standard-compliant inspection of primary packaging materials
The full vial inspection after filling and closing is then carried out by the spotter II PHS with a wide range of detection methods in a single device. And for blow-fill-seal containers sealed by melting, such as ampoules filled with small volume parenteral products or infusion bottles containing large volume parenteral products, the new but already tried and tested spotter II BFS realises a 100 per cent all-round inspection and leak test.
With personalised access rights and seamless recording of all relevant operating and process data, the cleanroom class B compliant Heuft systems for the inline inspection of empty and filled parenteral primary packaging materials, basic documentation and validation obligations in accordance with GMP and the valid guidelines for computerised validation (GAMP5).