Customised security concepts and labels for pharmaceutical packaging can help to curb problems with counterfeit, harmful or tampered medicines. Various solutions designed by pharmaceutical labelling specialist Schreiner MediPharm also make an important and effective contribution here.
For years, the pharmaceutical industry has been facing the Growing threat from counterfeit medicines exposed. This now not only affects developing countries and emerging markets, but has also become a global challenge. The risk of grey market activities and illegal reuse of original containers is also increasing. These counterfeit medicines can result not only in brand and image damage for pharmaceutical companies, but also in considerable economic losses. In addition, more and more regulatory requirements that require special solutions for product and brand protection and for tracking the supply chain. require.
Individual security concepts minimise counterfeiting potential
The introduction of the EU Falsified Medicines Directive in February 2019 was the first legal, Europe-wide measure to prevent the threatening development of counterfeit medicines. However, the directive does not take into account the primary packaging, but only the outer packaging of the medicine and also Characteristics for verifying originality are not prescribed, but are at the discretion of the pharmaceutical manufacturer. However, these play a major role in meeting all possible threat scenarios. The core issue is therefore Integrity and security of the entire pharmaceutical supply chain.
The first step in developing an effective safety concept is to determine the situation, for example what threat the medicinal product is exposed to and who will be involved in the verification process. If the threat lies in the exchange of medicinal products Security labels with first-opening indication that irreversibly indicate a tampering attempt and ensure the integrity of the packaging. Security labels with integrated overt, covert and digital anti-counterfeiting features are the ideal solution for checking the authenticity of counterfeit medicines. If the problem is illegal diversion, then Security labels with RFID/NFC and track & trace solutions for digital identification and online verification help to prevent the scenario. After analysing the requirements profile, pharmaceutical security experts work with the pharmaceutical manufacturer to define the individual security concept.
Security features protect primary and secondary packaging
In general, every safety concept must be tailored to the respective packaging format such as folding box, syringe or vial. The most effective are Concepts that combine different security features and can be supplemented with additional functions. This results in effective, multi-level security solutions for tamper evidence and first-opening indication, counterfeit protection and proof of authenticity as well as identification and traceability.
Void seals, for example, such as the one developed by pharmaceutical labelling specialist Schreiner MediPharm. The special void effect becomes visible when the label is first removed and clearly and irreversibly indicates the initial opening.
„Depending on the application, the seal can be realised with three different void effects: No Transfer leaves no residue on the packaging, the void effect is only visible on the label after initial opening. Semi Transfer leaves partial residue on the packaging, the void effect can be seen on the packaging and label after opening for the first time. With full transfer, a complete layer remains on the packaging, the void effect can be seen on the packaging and label after opening for the first time. If additional overt or covert authentication features are integrated into the labels, they can also serve as proof of authenticity.“
Dr Nadine Lampka, Senior Product Manager Pharma Security at Schreiner MediPharm

The „Cap-Lock“ cap adapter solution, a combination of safety label and cap adapter for Luer lock syringes, is suitable for irreversibly signalling the initial opening of primary containers. „A cap is placed on the primary closure of the syringe to compensate for the different radii of the syringe body and closure. A label is then applied to enclose the syringe body and the lower part of the adapter. When the syringe cap is opened, a integrated perforation partially destroys the label and irreversibly indicates the initial opening“, explains Nadine Lampka.
Counterfeit protection features in combination
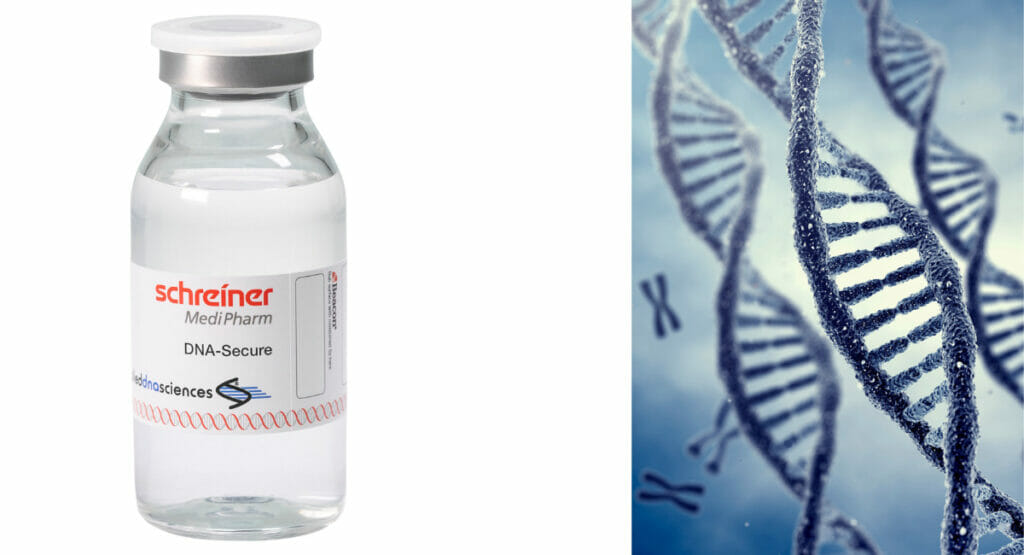
The variety and range of anti-counterfeiting features on the market is quite large. Who pharmaceutical safety, combines overt, covert and digital features with each other. A single authentication feature that is already highly secure is based on SigNature DNA - in cooperation with Applied DNA Sciences, Schreiner MediPharm has developed a Label with integrated individual DNA developed. It is one of the hidden features, is considered unforgeable and is recognised in court as forensic authentication evidence. The high-security feature is based on uniquely modified, encrypted DNA sequences that are invisibly integrated into a label. The short, customised DNA sequences are produced from plant material using biochemical methods. Various test methods are available to verify the authentication feature: In addition to special mobile PCR devices, a forensic DNA analysis can be carried out by a laboratory for test results that can be used in court.
If the focus is on identifying and tracing a medicine, digital seals with NFC technology and tracing systems are a good option. Both can be integrated into various label forms. One example is the Combination of a digital security feature and a first-opening indicator on a booklet label. The bottom layer of the multi-page label contains a closure seal with a void effect as an irreversible first-opening indicator. „In addition, a unique 15-digit alphanumeric code is printed on the label as a kind of fingerprint, with which the medication can be verified in real time on the Internet, with a smartphone or via a hotline. The label also contains an NFC chip that enables digital authenticity checks via smartphone,“ adds the expert.

Challenges for the next decade
Global supply chains for pharmaceuticals are becoming increasingly complex. Products manufactured in one country may be packaged in a second country and distributed across borders to be marketed or sold to consumers in a third country. With the growing trend towards e-commerce, it will be even easier to purchase medicines online in the future, but unfortunately often from unauthorised sources. The WHO estimates that more than half of the medicines bought online on illegal websites are counterfeit are. The WHO has therefore identified stopping this illegal trade as one of the most urgent challenges for the next decade.
More news from the magazine

Translucent paper seal labels for pharmaceutical packaging

Packaging machine manufacturer in flow

Powerful automation for mills

Decentralised signal processing with the Schmersal Safety Fieldbox