Since the start of the Covid-19 pandemic and the efforts to combat the virus, clinical trials have increasingly become the focus of public attention. However, without blinding, their results are often not meaningful. Labelling specialist Faubel knows the challenges that need to be overcome when it comes to the targeted concealment of information.
Research on Covid vaccines is sometimes the subject of controversy due to their accelerated implementation, without the basics of clinical trials even being known. In general, every drug must be approved by the authorities before it enters the market. Authorisation procedure in which efficacy, quality and safety must be proven by means of preclinical and clinical tests. In order to prevent unconscious falsification of the test results by the study participants, the study physicians and the nursing staff in charge, a Blinding indispensable.

Which authority is responsible for the clinical trial depends on the specific product and the countries in which the pharmaceutical company wishes to launch the medicine. In the European Union, authorisation can be granted centrally via the European Medicines Agency or nationally. In Germany, the Federal Institute for Drugs and Medical Devices (BfArM) or the Paul Ehrlich Institute (PEI) responsible for vaccines and blood products.
In Germany, the requirements for Drug authorisation in the German Medicinal Products Act (AMG) are defined. The Medicinal Products Act also authorises the legislator to lay down further regulations. One such regulation is the GCP Regulation, which regulates the requirements for clinical trials with regard to principles, guidelines and the application of good clinical practice.
Multiple phases, double fuse
Frank Jäger, who is a member of the management of Faubel Group who is also responsible for product development and new technologies, describes the Ideal case of a study, „if neither the study participants nor the study physicians or the caring nursing staff know which group the patients have been assigned to“. This approach describes a double-blind study approach.
Initially, the tolerability of the drug or active substance is investigated in phase I with healthy participants. In phase II and III trials, efficacy and tolerability are first tested in a small number of patients and then in a large number of patients. The approval of the drug after phase III is usually followed by phase IV trials, in which comparisons are made with other drugs, for example.

Well thought-out concepts of blinding and labelling are a prerequisite for usable results in clinical trials.
Frank Jäger is Managing Director for Product Development and New Technologies at the Faubel Group, Melsungen.
The patient collective, also known as the intervention group, receives the new active substance. In contrast, a control group, patients with the same disease or the same disease risk, are given no medication, an ineffective dummy medication (placebo) or the current standard medication. In addition to the random and unpredictable allocation to the treatment groups (randomisation) blinding prevents unconscious falsification of the study results. The processes in this context are very complex.
Labelling specialist Faubel has been supplying the pharmaceutical industry since it was founded in 1855. „It was a logical step for us to specialise in the Labelling of clinical studies These labels are subject to high quality standards and are often more complex in terms of the number of pages and the function of the labels. Our pharmaceutical customers value our experience and expertise, which means that the Clinical Trial Labelling around 70 per cent of our sales makes the difference,“ as Jäger emphasises.
Labels as all-round talents
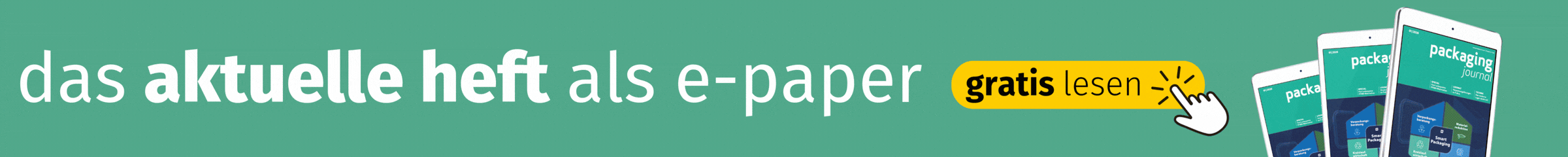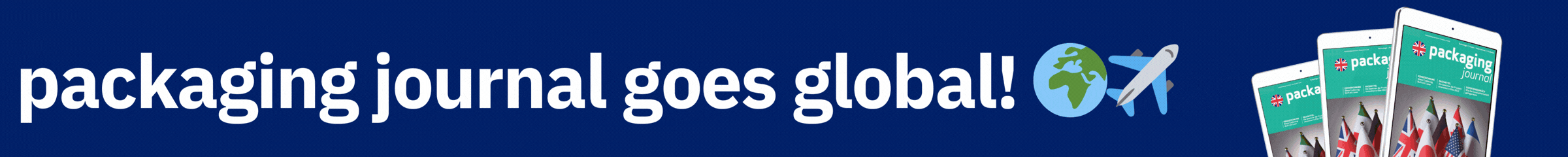
One Blinding makes the packaging, the medication and the placebo look the same. According to Frank Jäger, this poses various challenges: „We usually produce blinding for vials, blister packs, tubes or syringes. In particular, containers where the application requires the original packaging require a customised blinding and labelling concept.“
Many of these customised concepts are labels. Labels blind syringes, blisters, tubes and inhalers and can label them at the same time. The design, format and number of pages are flexible and the The properties of the material and adhesive can be adapted to the filling, storage and transport conditions. „For example, special films, strong adhesives and resistant colours can withstand high humidity during application, or these labels can be frozen together with the medication,“ says Jäger. The labels cover the primary packaging so that it is opaque and impermeable to light.

In multinational clinical trials, various national languages often have to be taken into account. The Use of booklet labels is obvious for Frank Jäger. „A few years ago, a customer commissioned us with the blinding and labelling of a small tube. Our product development team created a large-sided booklet label with a larger base label. The base label blinded the tube and the booklet label offered enough space for different languages in a reader-friendly font size.“
This clinical trial was double-blind; the allocation of active substance, placebo and standard medication was unknown to the study participants and the study physicians. The booklet label revealed the study, kit and patient numbers as well as the expiry date. It also contained information on how to use the ointment, how to store the tube and the contact details of the sponsor of the clinical trial. According to the documentation provided to the physicians by the study coordinator, each tube could be clearly assigned. In the event of a medical emergency, the patient number and the randomisation list could be used to trace the allocation.
Alternative blinding and 3D printing
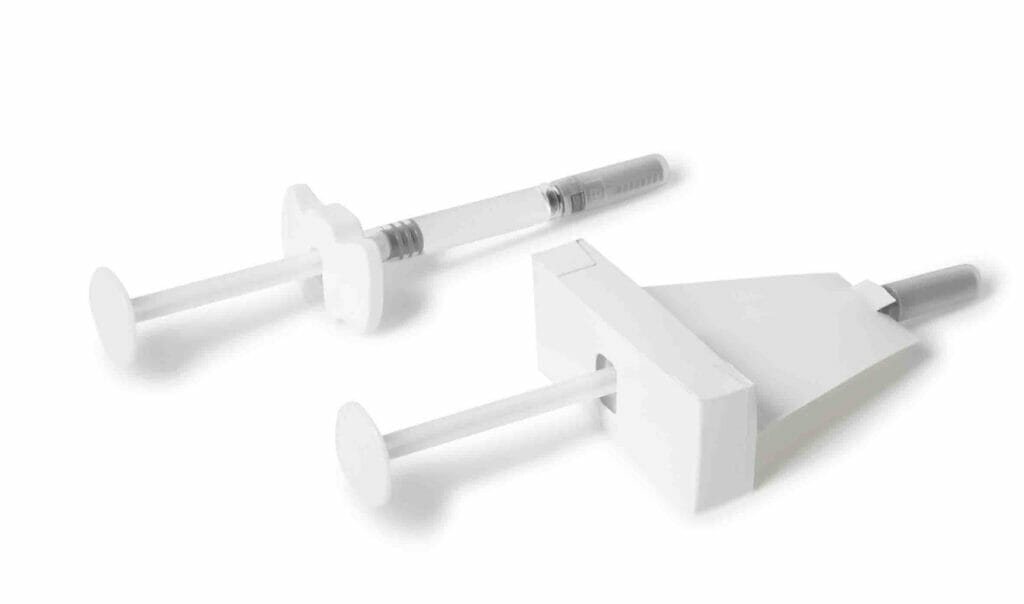
A label is not always suitable for blinding. If the height or circumference of the primary packaging differs from one another Boxes made of sturdy and opaque cardboard for full-surface lamination. Supplemented with an insert, the differences are invisibly evened out. „If the box encloses a vial, the injection needle can be inserted easily thanks to a punched-out hole. An adhesive film protects the injection site from external contamination. This is particularly useful if liquid is injected several times from the same vial,“ explains Jäger. A combination of box and foil is also useful for infusion bags and bottles. An integrated foil flap makes it easier to hang them up.
Nowadays, the 3D printing process enables the production of highly customised containers. Instead of cardboard, the box can also be made of plastic: These include versions that have a punched-out inspection window. If the liquid is cloudy, doctors and carers can remove the spoiled medication directly from circulation.
The plastic gives the veneer enormous stability and offers a high degree of variance for very small series.
Sophisticated concepts reduce costs
„As a rule, a lengthy and cost-intensive process is required before a drug is ready for the market. On average, it takes more than ten years, the development costs amount to around two billion US dollars, and only just under nine per cent of active substances ever reach market maturity.“ These figures influence Frank Jäger and his team when they develop blinding agents. „Well thought-out concepts reduce effort, costs and time of clinical research. It was not only through Covid-19 that we all learnt that the time factor can save lives. As a supplier, our company is aware of this responsibility.“
Magazine articles


Traffic lights prevent bottlenecks

Three applications, one solution
Translucent paper seal labels for pharmaceutical packaging

Packaging machine manufacturer in flow