Schreiner MediPharm is presenting a solution for blister-free packaging of prefilled syringes at Pharmapack. Together with partner companies Schott Pharma and Körber Pharma, the company is demonstrating how sustainable packaging design can combine waste and cost reduction with user convenience and patient safety.
The new solution is based on Cap-Lock, a new type of functional label from Schreiner MediPharm which, in combination with the Schott Toppac infuse COC syringe and an innovative special cap, can completely replace the previous blister packaging. Thanks to a new cap design, which has the same diameter as the syringe body, the Cap-Lock label can be applied to the syringe with process reliability. At the same time the functional label encloses the syringe barrel and cap like a second skin and offers an irreversible first-opening indication that is automatically triggered when the cap is opened.
This functionality ensures the integrity of the prefilled syringe. from production to end use secure. This prevents tampering and misuse, and end users can immediately recognise whether the syringe and label have already been opened.
In addition to the first-opening indication, the Cap-Lock label takes over additional protective functions of the conventional blister and transfers them to the primary container: A gas barrier and multi-level UV and light protection can be integrated into the label design. The extended label area allows flexibility in terms of individual branding, product information or medication colour codes. Also an RFID chip for automated tracking at unit level and for digital first-opening indication can be integrated into the Cap-Lock label.

The Cap-Lock label from Schreiner MediPharm encloses the syringe and cap and irreversibly indicates the initial opening. (Image: Schreiner MediPharm)
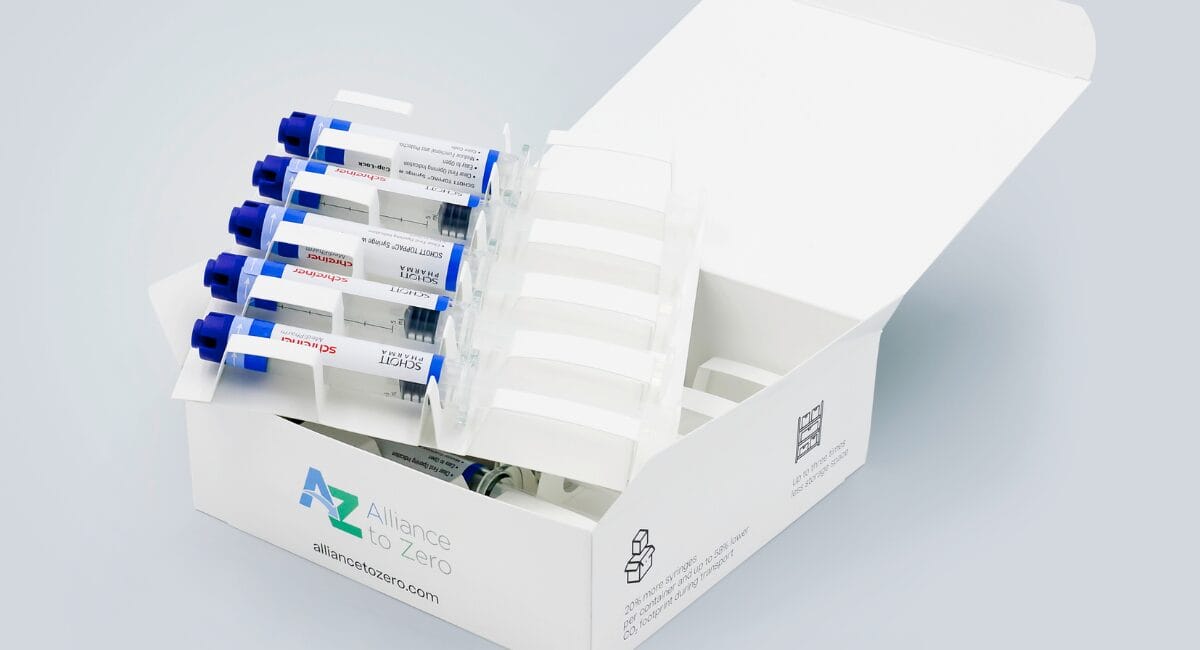
The combination of COC syringe with special cap and functional label with sealing function means that the filled syringes can be used in volume-optimised top-load carton packaging made from 100 per cent mono-material from Körber Pharma. This ensures optimised packaging and distribution processes in pharmaceutical production and significantly less space is required for transport and storage.
The new packaging system therefore not only offers considerable cost savings for pharmaceutical manufacturers, but also makes a significant contribution to sustainability. Thanks to the elimination of blister packs, the 5 ml Schott Toppac infuse 1,260 more syringes transported per pallet This results in a saving of 16 containers for ten million syringes. The elimination of blister packaging reduces plastic and plastic waste by 80 per cent.
The system consisting of a prefilled COC syringe, special cap and Cap-Lock label in combination with the cardboard packaging has already been underwent and successfully passed an ISTA-3A transport test. The three partner companies as Founding members of the Alliance to Zero thus support the path to reducing the CO2 footprint along the value chain and at the same time ensure user comfort as well as product and patient safety.
Source: Schreiner MediPharm
Schreiner Medipharm at the Pharmapack in Paris: Stand C70