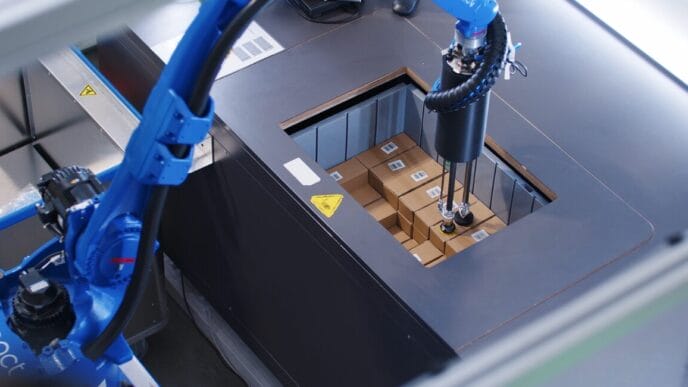
Vaccines against viral diseases are among the most successful inventions in the history of medicine and are currently very popular. The biotechnology company Bavarian Nordic is using state-of-the-art technology for its new isolator filling system for freeze-dried and liquid vaccine sera.
In the biotechnology sector, the number of companies producing vaccines is increasing. But the production systems they use are also changing. There are also numerous new challenges in this area: vaccines have to be developed, produced and brought to market in a short space of time. At the same time, it is important to keep process costs low without jeopardising the safety of patients and employees.
Benefit from years of experience
Thanks to 25 years of experience in the pharmaceutical market, the Biotech pioneer Bavarian Nordic recognised that change and innovation are key factors in remaining competitive in a volatile market environment. In view of the growing demand for vaccines, the company decided to expand its capacity and build a new production facility at the Kvistgård site in Denmark. In order to meet the industry-specific challenges and developments, the new plant had to have a complete isolator filling line for liquid and freeze-dried substances.

„On the one hand, we wanted to develop a line for processing liquid products and for freeze-drying active ingredients in order to extend their shelf life,“ explains Bo Seligmann, Head of Product Support at Bavarian Nordic. „On the other hand, we also wanted to expand our capacity for contract filling with the new line.“ After weighing up all possible options, the company decided to source all components for the filling line from one supplier: „Due to the complex requirements of this project, we wanted to harmonise all machines and processes as smoothly as possible,“ explains Bo Seligmann. „Syntegon Technology's line expertise was exactly what we needed.“
Performance and safety go hand in hand
The decision was made in favour of a Isolator filling system for vials from Syntegon Technology (formerly Bosch Packaging Technology) in combination with the integrated freeze dryer from a third-party supplier. The highly complex isolator line consists of nine machines and complies with GMO protection level 2 (BSL2). This is considered the standard for production systems that work with attenuated live vaccines.
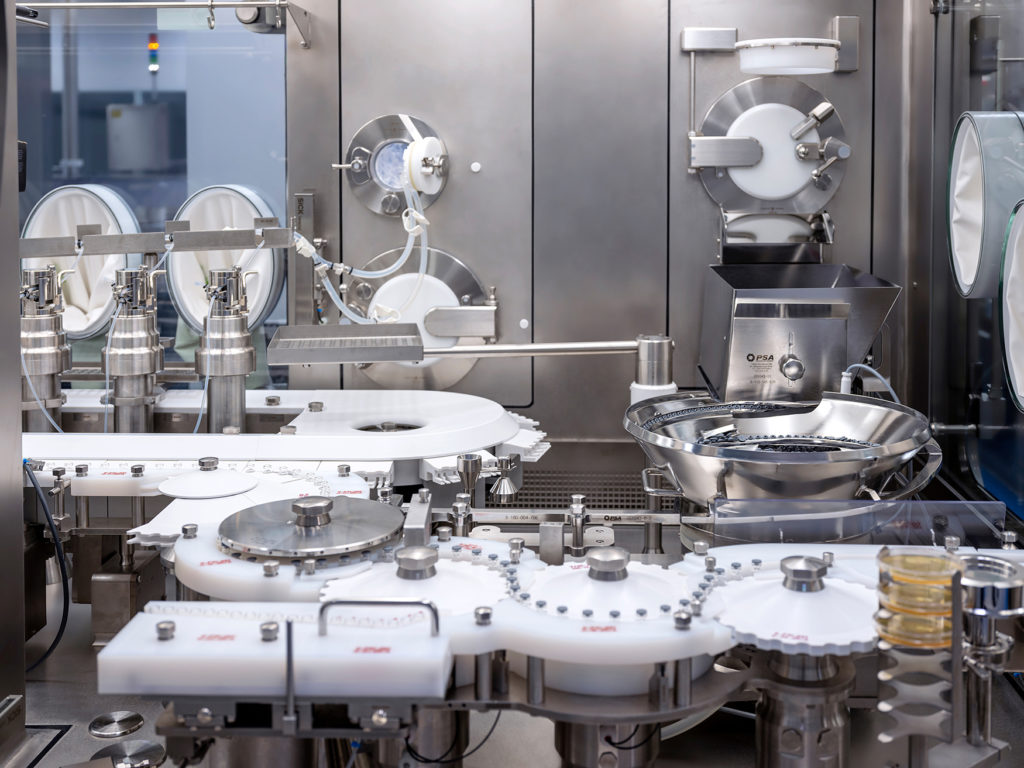
The MLF 5088CS filling machine forms the centrepiece of the line. This ensures a high output of 400 containers per minute with the highest safety standards and an integrated one hundred per cent in-process control (IPC). The filling machine is equipped with the patented PreVAS disposable dosing system, which reduces complicated cleaning processes and minimises the risk of cross-contamination. The entire system is fully integrated into the isolator filling line so that the operator and product do not come into contact with each other.
The line also includes a cleaning machine from the RRN series for flexible and optimal cleaning of the vials, an HQL sterilisation tunnel for safe and reliable sterilisation and depyrogenation of the pre-cleaned containers and a downstream capping machine from the VRK series. The filled and capped vials are cleaned by an external cleaning machine from the RAN series. This ensures that no particles adhere to the outside of the containers - an important factor in protecting operators and medical staff from active viruses.
An ISS-H2O2-transfer lock ensures that the material enters and leaves the isolator quickly and without contamination. As a stand-alone machine, it can be biodecontaminated independently of the current isolator status. In addition, the isolator line is decontaminated twice during production: the first time as a standard process before the start of production and a second time immediately afterwards to inactivate any product residues still in the line.
„It was important for us to use reliable and proven standard machines in order to guarantee high product quality.“ Bo Seligmann, Head of Product Support at Bavarian Nordic
More efficient production thanks to parallel processes
In addition to using reliable and proven standard machines to ensure high product quality, the line had to be as compact as possible to save space and as flexible as possible to fill different container formats. The machines from Syntegon Technology also allow quick format changes. The line is also designed so that Bavarian Nordic can carry out different processes at the same time. As the freeze-drying process sometimes takes several days, Bavarian Nordic uses the line to fill liquid products in the meantime.

However, efficiency is also a question of time: as Bavarian Nordic fills live viruses, the company has to process the sensitive liquid ingredients quickly. „This means that the new machines have to function quickly and absolutely reliably,“ says Bo Seligmann.
Bavarian Nordic receives the active viral sera in frozen form in order to thaw them to around five degrees and then fill them. During the entire filling process, care is taken to ensure sufficient cooling so as not to compromise the integrity of the active ingredient. The Syntegon experts make it possible to fill chilled products at between two and eight degrees Celsius by reducing the humidity inside the isolator.

A successful premiere
The technically demanding line arrived on time at the Bavarian Nordic site in Kvistgård. The operational qualification tests were completed just seven months later. „Syntegon Technology kept to the agreed schedule and our colleagues worked with us in a committed and constructive manner,“ emphasises Bo Seligmann.
A thorough risk assessment by Bavarian Nordic and Syntegon Technology and a comprehensive alarm and function test at Syntegon Technology's site in Crailsheim ensured that every single machine worked as expected.

The experts from Syntegon also supported Bavarian Nordic during the qualification phase. „Syntegon's employees know their machines best and carried out their work very efficiently. Completing this project in such a short time was only possible thanks to the professional co-operation between the experienced Bavarian Nordic project team, the Syntegon representatives and the third-party supplier of the freeze dryer,“ adds Bo Seligmann. „The collaboration with Syntegon Technology was a very successful première for us.“
[infotext icon] Bavarian Nordic is a fully integrated biotechnology company with sites in Denmark, Germany, Switzerland and North Carolina, USA. The biopharmaceutical manufacturer specialises in the development, production and marketing of vaccines[/infotext].