At ACHEMA, Optima Pharma is presenting CSPE 2.0, a concept with which complex turnkey projects consisting of a filling and capping system, isolator and freeze dryer can be realised to the point in order to achieve reliable production start-ups in a shorter time.
With CSPE 2.0, Optima Pharma is presenting the next development stage of the process in the Expert Zone of the trade fair, with which complex turnkey projects can be completed in less time and system integration is further deepened. A visible sign of this is the recently opened CSPE Centre II (Schwäbisch Hall) with space for filling and capping systems, isolators and, for the first time, freeze-drying systems as a complete system from a single source.
As a further innovation, Optima Pharma can carry out extended qualification measures in-house, including significant parts of the cycle development. This significantly reduces the time required between installation and the start of production at the customer's site. The new product presented at the ACHEMA The process presented also includes process simulations of the entire system during the design phase through to integrated factory acceptance tests (iFAT) in the CSPE centres. At this point, complex turnkey projects have already been comprehensively tested under realistic conditions.

Premiere of the new generation of Optima MultiUse systems
Today, digital technologies are an indispensable part of highly efficient fill & finish processes. The comprehensive digitalisation portfolio - the Intelligent Production Assistance Services (IPAS) - includes, for example, thehe continuous analysis of machine data and evaluations on the dashboard, to recognise fluctuations in performance at an early stage.
Predictive maintenance, which avoids unplanned machine downtime, is also part of this. However, if a service case does occur, the fastest troubleshooting is achieved remotely with secure, digital access. The IPAS portfolio from Optima Pharma also include augmented reality solutions to visually guide the system operator's service staff through maintenance procedures and support them with information in data glasses. Flow and other types of simulation are used in the design phase. Virtual reality accompanies the realisation phase, for example with virtual mock-ups in Optima's Digital Innovation Center.
Premiere: The MultiUse machine series has been completed. The latest generation of Optima MultiUse machines processes up to 24,000 containers per hour. Optima Pharma is the first company to offer machine solutions with identical functions from the laboratory to the high-performance range. Processes can thus be transferred one-to-one to high performance. This includes, for example, product-saving functions such as re-dosing and re-capping, which have proven their worth with high-quality to very expensive medicines. The extremely high filling accuracy of the MultiUse machine concept is also maintained right up to the high-performance range.
The flexibility of the MultiUse systems will also be a topic in the Expert Zone: not only can the container types syringes, vials and cartridges be processed largely without format parts, but it is also possible to use the MultiUse systems in a wide range of applications. different processing paths can also be integrated into one machine system. For example, a path for ready-to-use containers (RTU) can be provided in parallel to a path for bulk containers. In addition to a path for processing liquids, a freeze-drying path can also be used. Part of the MultiUse system is a sophisticated robotics concept that utilises the strengths of different robot types in a targeted manner, for example to compensate for potential empty positions when renesting containers in trays.
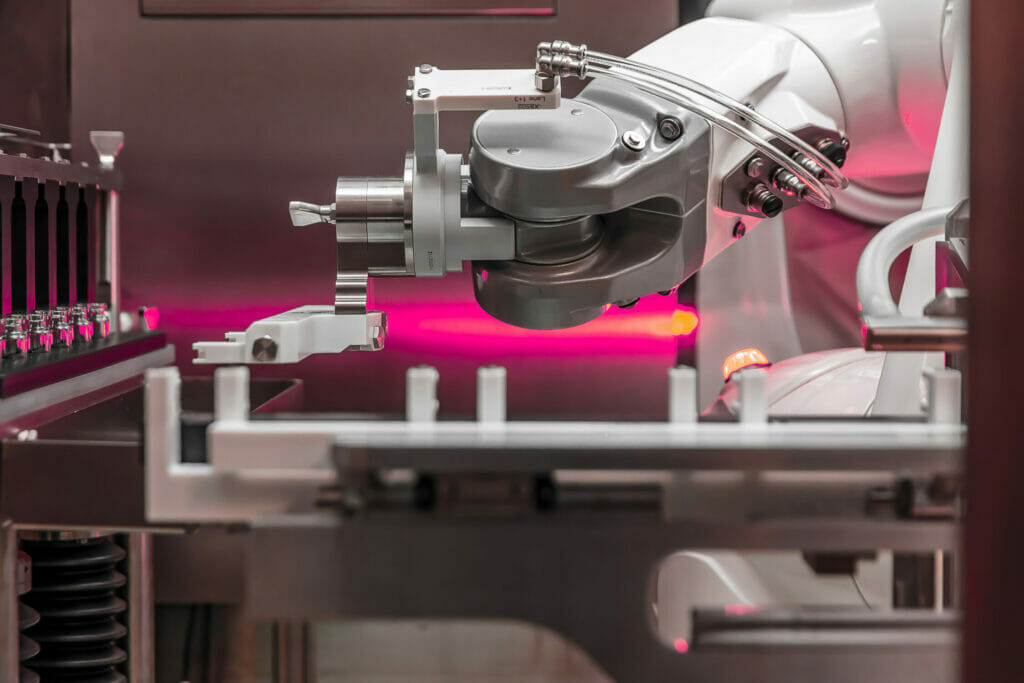
Technologies for new types of active ingredients
Pharmaceutical innovations such as cell and gene therapies require new approaches to sterile processing. In close partnership with leading clinics, Optima Pharma System concepts for highly reliable and flexible manufacturing processes which help to significantly reduce the currently still high costs of the new precision medicine. The Expert Zone will show how automation concepts and robotics can significantly reduce the proportion of manual tasks and increase pharmaceutical safety at the same time. Innovative solutions for filling particularly high-quality, expensive drugs such as viral vectors are another topic here.
Many new active pharmaceutical ingredients are considered highly potent. At the ACHEMA, how specific system concepts optimise the safety of the operating personnel and at the same time ensure the integrity of the medicinal product, for example, by using special transport systems to effectively prevent potential carry-over of active ingredients. In addition, the advantages of turnkey projects in the processing of highly active ingredients are realised when filling and capping technology, isolator technology and freeze-drying are designed as a complete system. Comprehensive system simulations, harmonised interfaces and integrated filter and washdown concepts for the filling line and isolators are examples of how process reliability can be increased and systems can be created from a single source.
Sustainability is also becoming increasingly important in pharmaceutical engineering. With a holistic climate and environmental strategy Optima Pharma to minimise energy consumption both in the operation of the machines and in their manufacture.
Source: Optima Pharma
Optima Pharma at the ACHEMA 2022: Hall 3.0, Stand A73
Optima - More news


Optima has expanded its presence in India

SIG and Optima join PulPac

Packaging Valley presents Fachpack appearance
Experience technologies live