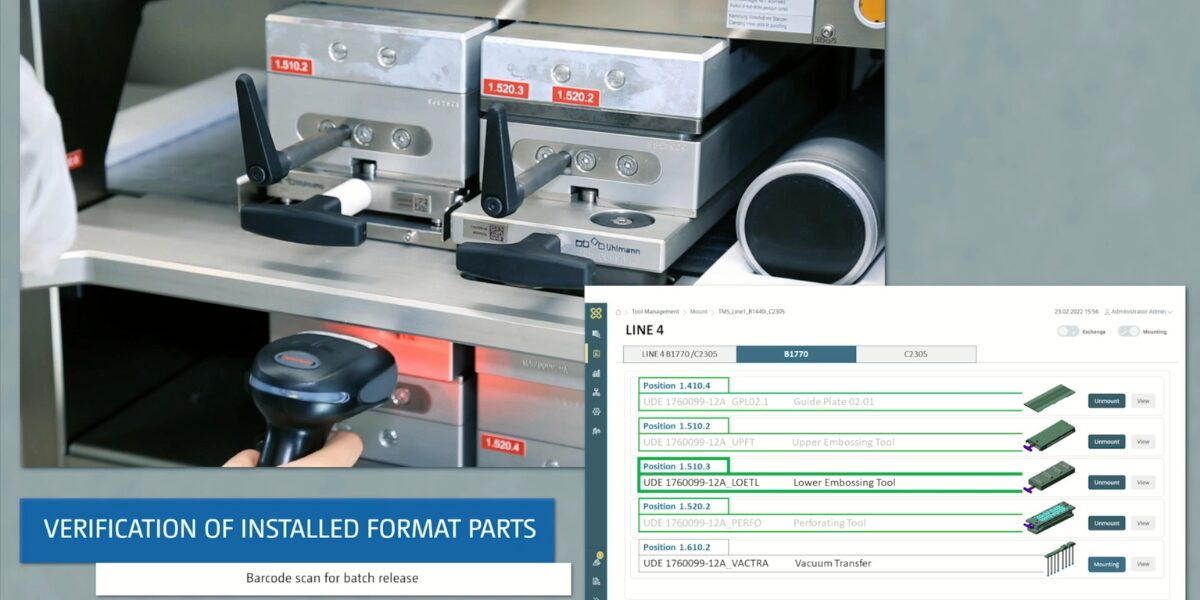
When pharmaceutical products are manufactured, there are special requirements for the tools. Safety and quality are essential. Tool management provides an overview of the life cycle of tools, which can range from simple Excel lists to intelligent software solutions and records the tools from the individual output to the interface to the ERP.
In most industries, the requirements of users and clients determine the type and scope of tool management. In the pharmaceutical and medical technology sectors, high regulatory requirements also play a role and make tool management more complex and costly. The pharmaceutical packaging expert Uhlmann Pac-Systems offers innovative, digital tool management solutions with which you can packaging processes more efficient, sustainable and legally compliant - regardless of the manufacturer.
Legal requirements and efficiency
Efficiency plays a decisive role at the economic level of a company, for which digital tool management is essential. The complexity of the stocks to be managed here is considerable. The size of a pharmaceutical packaging company's tool and format inventories naturally depends heavily on its specialisation. Stocks of around 30,000 tools are common. In order to keep these quantities of tools under control, the tool management specialists at the packaging expert Tool and format management services to. This is where the Pexcite software platform comes into its own. Track & trace solutions are often the first step towards the digitalisation of packaging processes.
Digital tool management does not simply replace paper with digital storage space: it offers possibilities, Automate and optimise processes to a greater extent. This makes the management of tools and moulds more stable, safer and more effective. The mould planning process, for example, is reduced to just a few seconds. In addition, the entire documentation effort can be optimised by over 60 percent and tools can be assigned to digital processes. A digital safety system actively avoids bottlenecks in production so that the entire process chain can be optimised. Effectiveness gains in the five-digit range per year can be achieved.
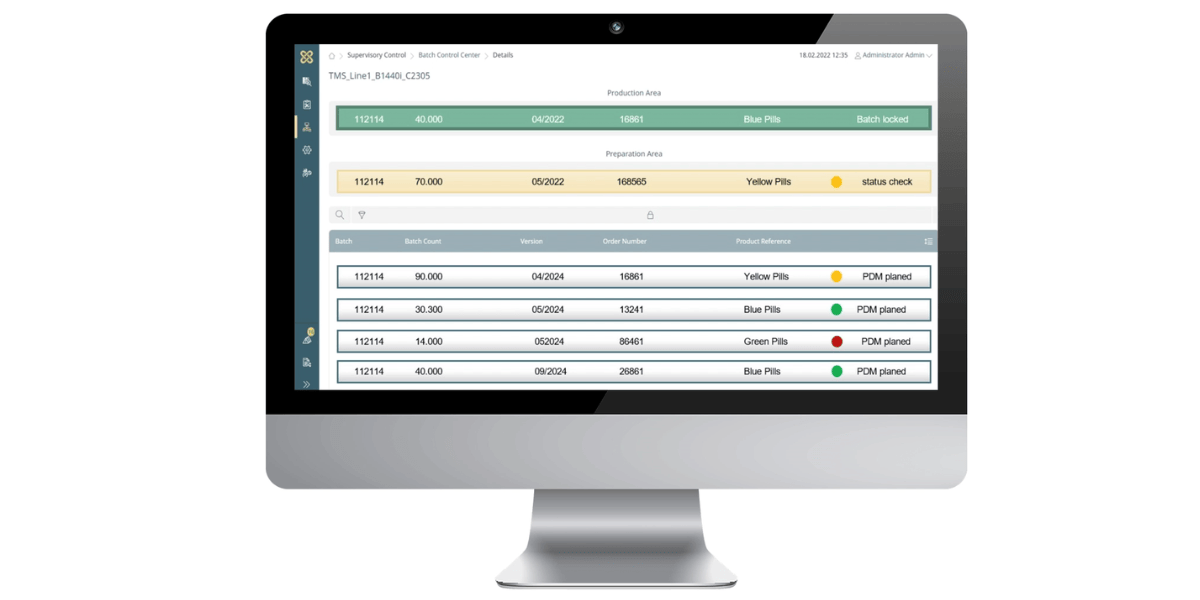
These advantages not only have an impact on efficiency. Compliance with regulatory requirements such as the EU GMP guidelines for human and veterinary medicinal products or 21 CRF Part II of the United States Food and Drug Administration also have a far-reaching influence on the type and scope of tool and format management in pharmaceutical packaging. This is why digital solutions for tool management are also crucial here in order to minimise the amount of Maintain tools in compliance and organise them safely.
Each of these tools and formats must be recorded and documented individually. It must be possible to clearly assign all data to this specific mould. In addition to typical data such as dimensions and functional data as well as removal and application data, details and information on whether and for which production systems and for which individual products the mould or format is qualified and validated must be stored. The digital processing of all this data enables efficient management and easy access to every tool required. However, when in doubt, detailed storage also has a legal dimension. This is because in the event of a recall or damage, manufacturers and packagers of medicines and medical devices must provide complete documentation of the specific production, packaging and delivery process of the product in question. In this way, the legislator ensures that the error can be identified and, if necessary, those responsible can be held legally accountable.
The data must match 100 per cent with the data available for process and availability planning, the retooling process and quality management. Even with a tool inventory in the lower four-digit range, this results in an enormous Organisation and documentation effort for the packaging company.
Trend towards more flexibility

Global trends in the medical and pharmaceutical industry, such as the individualisation of therapies and CO2-The increasing complexity of processes in tool and format management in recent years has led to a rise in the number of new and neutral tools. How this is handled already has a significant impact on profitability. After all, the Set-up times of a packaging line approx. 30 per cent of the overall line efficiency (OEE).
Thanks to digital tool management solutions, packaging companies can meet these requirements in a more flexible and modular way and better fulfil customer wishes in addition to complying with regulations. In addition, the Set-up times significantly reduced compared to pre-digital times.
Implementation expertise thanks to digital solutions
The information generated by the digitisation of inventories can also be analysed according to various aspects so that processes can be optimised or redesigned. Employees can use their computers to access the system and the functionalities that have been activated for them. In production, access is also possible via tablets. One Intuitive user interface makes cross-shopfloor tool management simple and usable for all employees. This avoids errors in the provision of tools. By storing the important data and individual steps, employees are guided safely through the changeover process and any risks are identified immediately. The life cycle of each tool and the usage history are automatically recorded and documented in such a way that they can be traced at any time, for example as part of quality management processes. In this way, digital tool management offers a cross-device solution with which errors can be avoided and which can be quickly applied and implemented by employees.