When pharmaceutical manufacturers want to launch new drugs on the market, the period between approval and market launch should be kept as short as possible. The uncertain success of a product also leads to uncertainties when making investment decisions for packaging capacities. Faller Packaging offers pharmaceutical manufacturers a suitable solution with the PrePackaging Service.
Pharmaceutical manufacturers have to provide packaging that meets all typical industry requirements before a drug can be approved. When it comes to market launch, every day counts. The sooner a product is available everywhere, the faster patients benefit and the high development costs pay off. However, the production of pharmaceuticals must be quickly adapted to the increasing demand. If manually assembled packaging is sufficient in the beginning, an automated solution is required as soon as possible after a successful launch. However, many months can pass before a new approval with machine-compatible packaging and the commissioning of a system. Conversely, manufacturers who build up a high production capacity before the launch bear a high investment risk. Packaging bottlenecks can also occur with established products, for example, due to unexpected market success or regional or seasonal fluctuations. Faller Packaging can support customers with all these challenges with its PrePackaging Service.
Tailor-made and far-sighted
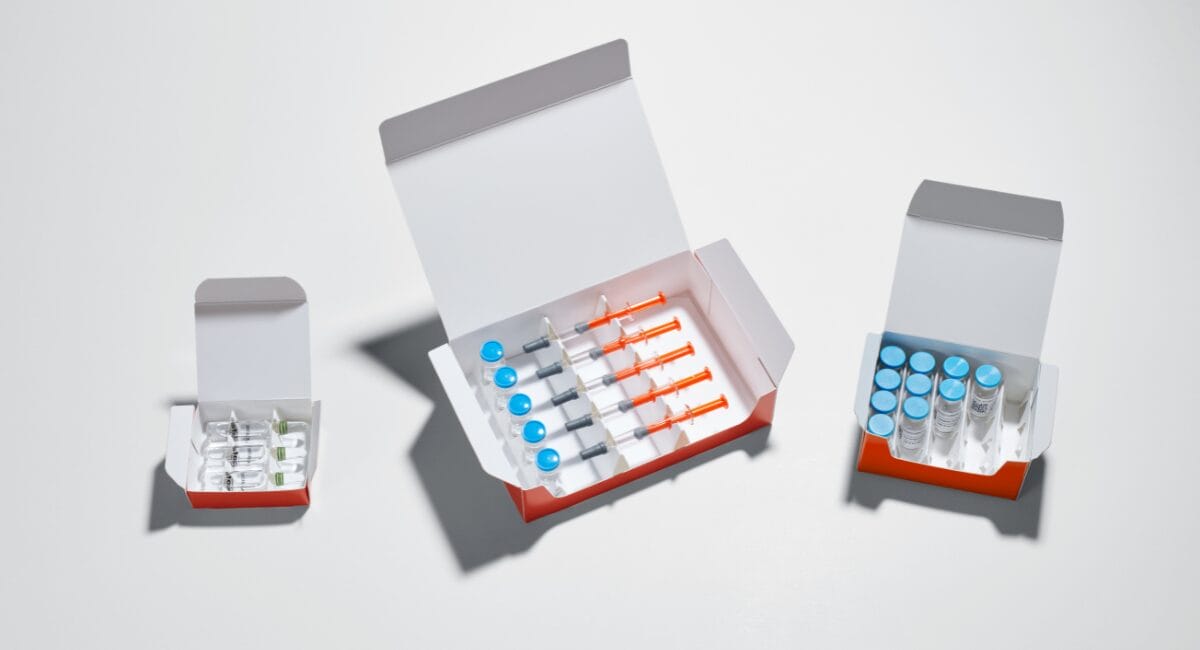
The specialist for folding cartons, leaflets, labels and combination products from a single source supplies pharmaceutical manufacturers with pre-assembled ready-to-use packaging that is precisely tailored to the requirements of the product. With the PrePackaging Service, Faller Packaging accompanies its customers from launch to series production and provides support for rapid market entry after approval. Together with the customer, pharma-compliant and recyclable carton packaging is developed for all parenterals and various dosage forms such as vials, ampoules, syringes, medical devices or combination products. Faller’s development department supports the customer from Clinical Trial Phase III onwards and works closely with them to find the optimum packaging solution for the product. The concepts are machine-compatible and can be used for both small and later large quantities without modification. They therefore cover the entire life cycle of the product and support the customer’s sustainability goals.
Manual, partially or fully automated and sustainable

Faller Packaging produces small quantities or complex packaging constructions by hand. As demand increases, the company produces the packaging semi-automatically. The folding boxes are set up by machine. Large quantities are produced fully automatically. In addition to ready-to-use folding cartons, the company also offers its customers additional packaging materials such as leaflets and labels. The highest quality, safety and product protection are essential for all concepts. Faller also implements these requirements as sustainable solutions in its PrePackaging Service.
The company uses certified inks and varnishes for printing. Paper and cardboard come from responsibly managed forests. The packaging is made from a single material, making it easy to close the recycling loop. Sustainability always includes the optimal use of materials. The aim is to use as few raw materials as possible and to avoid cavities, for example. This is why Faller Packaging is constantly working on new solutions and designs. It is also essential that the materials are easy to recycle.
Seamless Packaging Service
Together with Schubert-Pharma, the specialist for toploading packaging machines, Faller Packaging also offers the Seamless Packaging Service, which goes hand in hand with the PrePackaging Service. The partners plan and implement packaging processes from manual to fully automated – tailored to the customer’s requirements and scalable to the required quantity.

In the start-up phase, pharmaceutical manufacturers only need small quantities. The pre-assembled packaging can be easily filled by hand at this stage. If demand increases in the intermediate phase, this is no longer possible. A cobot module is then used to automatically fill the already erected folding cartons with the primary packaging and the package insert. If demand continues to rise, fully automatic toploading machines will be used for series production.
The better the machine and the ready-to-use packaging are coordinated, the more efficient and reliable the automated packaging process will be. It is therefore important to plan the secondary packaging – i.e. folding cartons, leaflets and labels – at an early stage in the development of a new medicine. This ensures that pharmaceutical manufacturers, together with Faller Packaging and Schubert-Pharma, not only reduce the time-to-market but also package more safely and cost-effectively throughout the entire product life cycle.