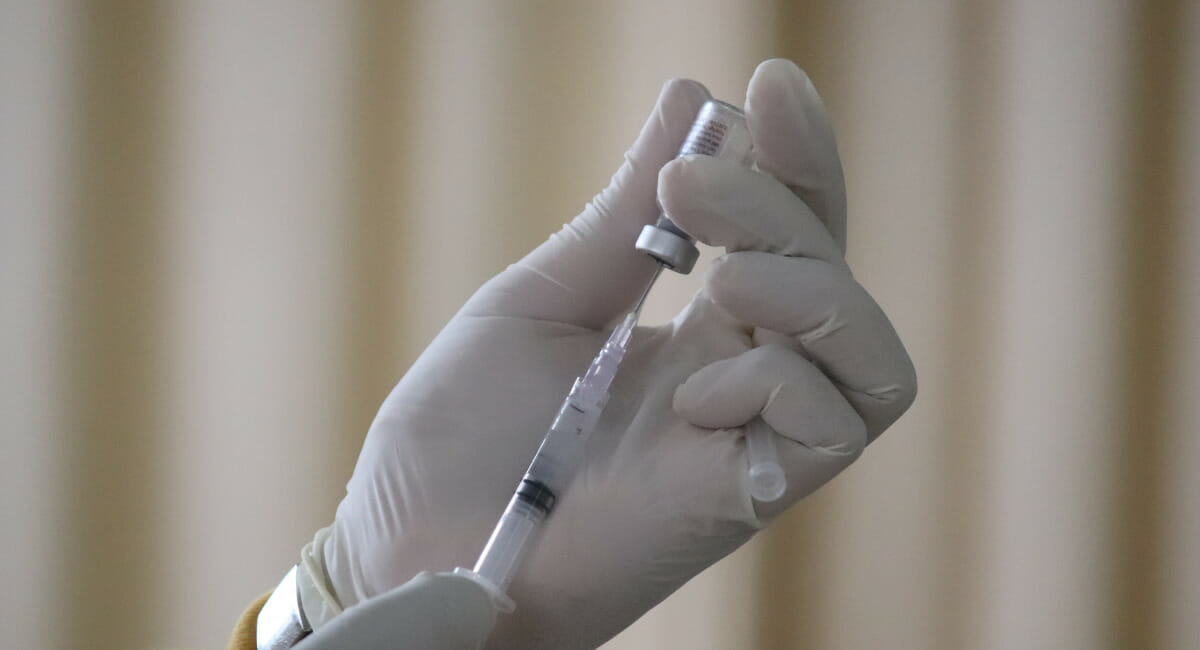
Pharmaceutical stoppers are often used for injection and drug vials – an extremely sensitive area with stringent material demands. Today butyl rubber is still being used as a standard material for manufacturing stoppers. During material production, certain degradation and reaction products can occur which then make their way into the end product. So a look at alternative materials, such as thermoplastic elastomers (TPE), is all the more worthwhile.
TPE materials are known to have the same soft-elastic properties as butyl rubber. However, thermoplastic elastomers offer even more advantages compared to the standard material used previously: TPE compounds are free of phthalates, harmful heavy metals, Latex and plasticizers. The material can also be processed easily using the widespread injection molding method. To produce stoppers out of rubber, however, a much more complex and laborious process is needed – and, at a time when cost-effectiveness and sustainability are becoming increasingly critical, this is a factor that should not be under-estimated.
Actega has developed the 6145 TL, 6245 NC and 6345 NC thermoplastic elastomers from the “ProvaMed” brand that have been created specifically for the requirements of injection and pharmaceutical stoppers and have therefore been exhaustively tested. These special TPEs have been compounded according to various material batches of different raw materials and fully comply with the requirements profile of both the European and United States Pharmacopoeias (EP / USP) in relation to safety, protection, function, and compatibility. To guarantee this, a range of mechanical and physical material properties have to be monitored in order to be able to ensure the materials’ ability to be sterilized and processed.
Proof of testing according to USP 381 (section on elastomer seals for injections) also has to be provided for injection or pharmaceutical stoppers. This proof includes verification of the physico-chemical tests as well as functional tests in which possible changes, influences and penetrability, self-sealing and fragmentation are all checked. Fragmentation and self-sealing in particular are of major importance for injection stoppers that are used for vials (drug ampoules) and which are pierced when the liquid the vials contain is drawn up using a cannula. Vials often contain multiple doses of a drug, which means that the stopper needs to be pierced several times. This, however, increases the risk of contamination and liquid leakage. To protect the drug remaining in the vial from such problems, the sealing material used must provide a reliable seal and protection, which must again be proven with appropriate functional tests.
Actega subjects the materials used to further tests, such as checks for non-intentionally introduced substances (NIAS), oxygen permeability or allergens. This is all made possible thanks to an in-house laboratory and a technical center equipped with injection molding machines and sophisticated application technology. The ProvaMed formulas achieve excellent test results in terms of sterilizability and biocompatibility according to ISO 10993-5 and exhibit 100 percent compliance with regulations. Pharmaceutical stoppers are sterilized in many different ways, such as autoclaving, EtO or gamma rays. The materials must therefore be suitable for treatment with all common sterilization methods without suffering any visual changes or mechanical stress.
With ProvaMed, ACTEGA now offers ready-to-use, functional and fully tested materials for the less complicated, more sustainable and more economical production of safe pharmaceutical stoppers. Renowned for its innovative strength, the company regularly invests a large amount in research and development, so that further innovations and advances in this and other areas of application can be expected in the near future.
Actega at Fakuma 2023: Hall B5 at Booth B5-5001